In late 2025, Eurachem published its new guidance entitled “The Fitness for Intended Use of Analytical Equipment and Systems” (AESQ). This guidance addresses the entire lifecycle of equipment and systems in analytical laboratories, recommending a risk-based and systematic approach to fitness assessment.
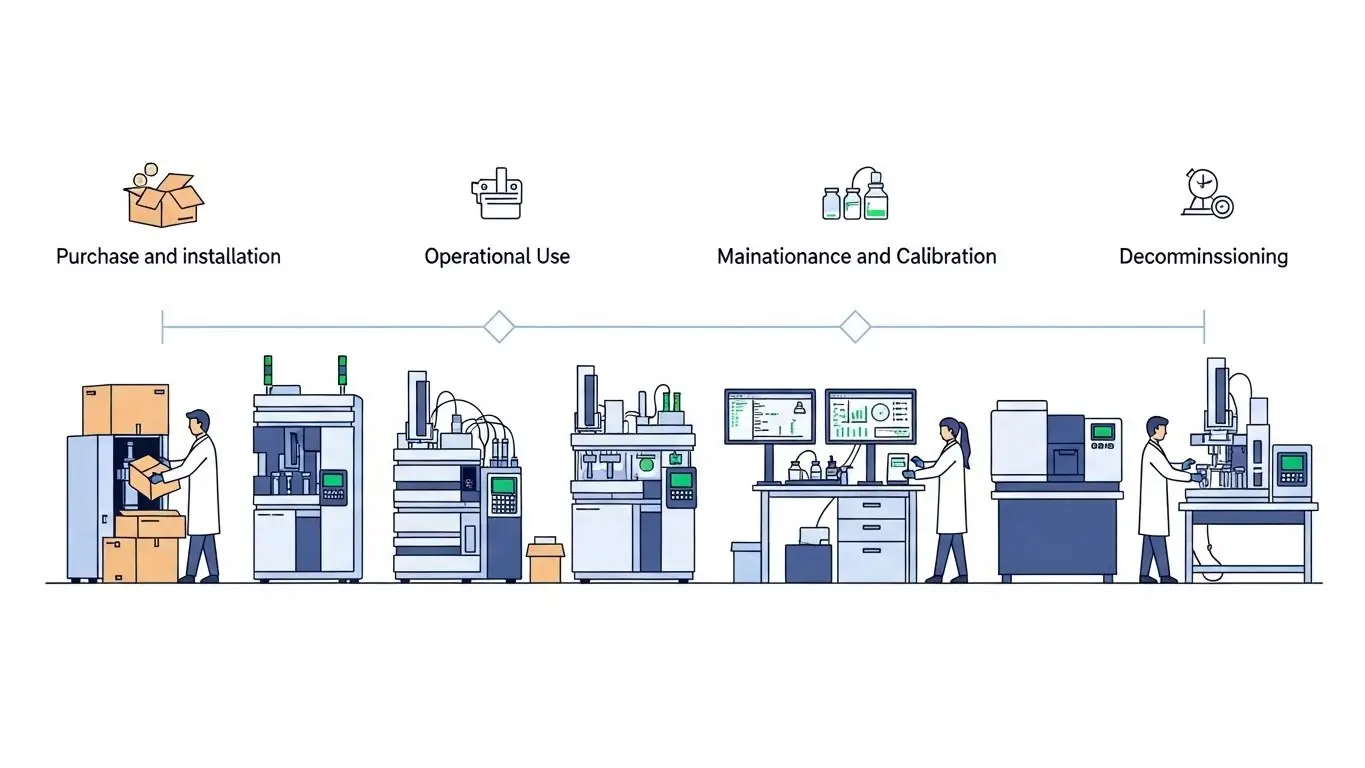
The comprehensive guidance covers all phases from equipment procurement through commissioning, use, maintenance, requalification and decommissioning. It also places special emphasis on critical issues such as equipment specifications, software control, data integrity (e.g. ALCOA+ principles).
The main objective of the guide is to ensure that laboratory personnel adopt common terminology so that validation, calibration, maintenance, etc. can be carried out efficiently. The guidance aims to strengthen laboratory quality and reliability by focusing on ensuring that equipment is ‘fit for purpose’, not just standards compliance.
As a result, the new Eurachem AESQ Guide offers a practical, comprehensive resource for quality managers, laboratory supervisors and technical teams in the management of analytical equipment. In particular for laboratories working to quality standards such as ISO/IEC 17025, the guide supports risk-based decision-making for critical decisions as well as meeting the standard across the entire equipment lifecycle.
For Detailed Information: Eurachem